Sweet
corn growers in New York, and elsewhere in the Northeastern USA, now need to
include managing northern corn leaf blight (NCLB) in their production program. 2012
was the first year NCLB occurred at a notable level in some areas, including on
Long Island. Growers were caught by surprise. Marketability of ears was
affected when symptoms developed on husks because it gave them an old
appearance, and the quality of the ear was affected. NCLB is expected to occur
in 2013 because this disease was common in 2012 and the fungal pathogen can
survive over winter in infested crop debris. Additionally it produces spores
easily dispersed by wind.
NCLB is not a new disease of corn (it was first reported in NY in 1878), but it had not been observed in some areas for many years. On Long Island it had been at least 20 years. It had been reported before 2012 as increasing in importance on field corn in the northeast and on sweet corn in New England. Increase in disease occurrence likely reflects change in the pathogen such that it is no longer suppressed by resistance genes in field corn varieties. Race 0 is thought to still dominant, but Race 1 has been detected; it overcomes the main major resistance gene, Ht1. Greater disease development in field corn results in more inoculum to affect sweet corn. Another factor may be storms that have been occurring during August with patterns that facility pathogen movement and disease development. NCLB and southern corn leaf blight are also known as Helminthosporium leaf blight, which is important to know because the later name is used on some fungicide labels.
The
pathogen produces an abundance of spores that are dispersed by wind.
Numerous spores were observed on spots examined microscopically. This
likely is how the pathogen moved to areas like Long Island
recently, and why the disease was widespread in 2012. A biologically
similar
pathogen, which causes southern corn leaf blight, moved from the Gulf
of Mexico
to Canada during one season in the 1970s.
Favorable conditions for the pathogen are moderate temperatures (64 - 81 F) and leaf wetness from rain, dew or fog for at least 6 hours. Conditions during August 2012 evidently were very favorable as that is when symptoms of NCLB were observed commonly in sweet corn plantings on Long Island.
Leaf spots are moderately large and long (1 to 5 inches),
elliptical, and grayish green becoming tan with age. Their shape resembles a
cigar or boat. Similar to rust, this
disease can impact ear quality when it develops on husks. Left unmanaged, NCLB
can develop rapidly causing a crop to become completely blighted and appearing
as affected by frost.
A primary reason to manage this disease is to minimize symptoms on husks, which can cause them to look old. Additionally, yield can be reduced when NCLB is severe. Grain yield of processed corn reportedly can sustain losses of up to 50% when the disease is established before silking; minimal losses in yield occur when the disease is delayed until 6 weeks after silking.
Cultural management practices include incorporating debris after
harvest and rotating crop land. However, since the pathogen produces spores
easily dispersed by wind, these practices may not contribute as much to control
of this pathogen as
for others that produce larger, heavier spores. The benefits to soil health of
reduced tillage in many cases will outweigh the benefits of reducing initial
inoculum by plowing in debris. Growing a resistant or less susceptible variety
is an effective practice. ex0876 7143 is resistant. Obsession was less severely affected by NCLB
than Beyond and ACR7196 in a variety evaluation
conducted in FL. More varieties exhibit some resistance in the large variety
evaluations conducted each year in IL (see Table ).
Providence appears to be among the more severely affected varieties based on
observations from commercial crops on Long Island in 2012.
).
Providence appears to be among the more severely affected varieties based on
observations from commercial crops on Long Island in 2012.
To determine when to apply fungicides for NCLB, each week inspect
crops thoroughly for symptoms, focusing on older leaves, and check for updates
on occurrence of NCLB in local extension newsletters. The potential for NCLB to
develop will increase with successive crops. Applying a protectant fungicide
(e.g. Bravo or Dithane) might be worthwhile when NCLB
has been reported in the area but symptoms are not found in the planting. Using
a spray boom with drop nozzles will increase spray coverage on leaves low in
the canopy, which is important because NCLB begins to develop there.
There are 2 groups of targeted fungicides effective for NCLB: FRAC Group 11 (which includes Quadris and Headline) and Group 3 (Bumper, Fitness, Proline, Propimax, AmTide Propiconazole, and Tilt). Corn is on a supplemental label for Proline. Targeted fungicides are more rain-fast than protectants and have a longer period of activity. Alternate among these groups and tank mix with a protectant fungicide to manage resistance developing in the pathogen. Starting early in disease development, when very few symptoms are present, is critical to successful control of most fungal diseases, including NCLB. The maximum number of applications that can be made to a crop is 6 for FRAC Group 11 fungicides, with no more than 2 sequential applications, and 2 to 4, depending on the product, for Group 3 fungicides. Other fungicides labeled for NCLB contain active ingredients in both FRAC fungicide groups: Headline AMP, Quilt and Stratego YLD. These are a good choice when only one or two applications are economical, as is often the case with field corn. The pre-harvest interval (PHI) is 0 days for Proline and Stratego YLD, which both contain prothioconazole. Stratego YLD also contains trifloxystrobin. PHI is 7 days for products with the other FRAC Group 11 fungicides. It is 14 days for fungicides with propiconazole as an active ingredient, which includes most of the FRAC Group 3 fungicides currently registered and the combination product Quilt. All these fungicides have a 12-hr REI.
Several
fungicide evaluations have been conducted recently in Florida where NCLB has
been an important disease in sweet corn for several years. Other foliar
diseases also occur there. The protectant fungicides tested exhibited similar
efficacy. Control was enhanced by using a spreader sticker. They were not as
effective as the targeted fungicides. The FRAC Group
3 fungicides (aka triazoles) were more effective than
the FRAC Group 11 fungicides (strobilurins) for NCLB;
the opposite was the case for rust. FRAC Group 11 fungicides were very good for
controlling NCLB at high label rates. Among the combination products, Headline
AMP was the most effective and Stratego was the
least, being similar in efficacy to fungicides with only a FRAC Group 11 active
ingredient.
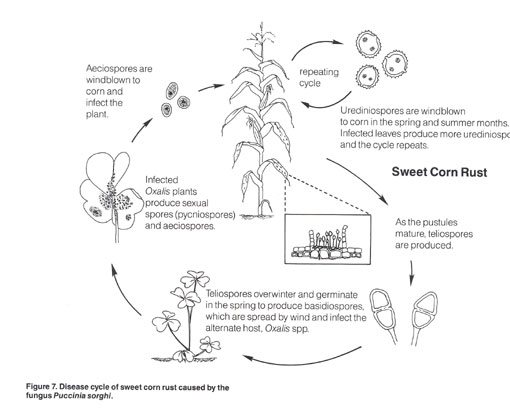
To effectively manage both NCLB and rust, growers should alternate among FRAC Group 3 and 11 fungicides, adjusting the program based on which disease is most important. Stratego is a good choice when an application is needed near harvest because the PHI is 0 days while it is 7 or 14 days for the other fungicides. Apply all targeted fungicides with a broad-spectrum fungicide for resistance management.
source : Effectively Managing Northern Corn Leaf Blight in 2013
Margaret Tuttle McGrath
Department of Plant Pathology and Plant-Microbe Biology, Cornell University
Long Island Horticultural Research and Extension Center; 3059 Sound Avenue
Riverhead, NY 11901; mtm3@cornell.edu
NCLB is not a new disease of corn (it was first reported in NY in 1878), but it had not been observed in some areas for many years. On Long Island it had been at least 20 years. It had been reported before 2012 as increasing in importance on field corn in the northeast and on sweet corn in New England. Increase in disease occurrence likely reflects change in the pathogen such that it is no longer suppressed by resistance genes in field corn varieties. Race 0 is thought to still dominant, but Race 1 has been detected; it overcomes the main major resistance gene, Ht1. Greater disease development in field corn results in more inoculum to affect sweet corn. Another factor may be storms that have been occurring during August with patterns that facility pathogen movement and disease development. NCLB and southern corn leaf blight are also known as Helminthosporium leaf blight, which is important to know because the later name is used on some fungicide labels.
 |
Favorable conditions for the pathogen are moderate temperatures (64 - 81 F) and leaf wetness from rain, dew or fog for at least 6 hours. Conditions during August 2012 evidently were very favorable as that is when symptoms of NCLB were observed commonly in sweet corn plantings on Long Island.
 |
A primary reason to manage this disease is to minimize symptoms on husks, which can cause them to look old. Additionally, yield can be reduced when NCLB is severe. Grain yield of processed corn reportedly can sustain losses of up to 50% when the disease is established before silking; minimal losses in yield occur when the disease is delayed until 6 weeks after silking.
 |
 |
There are 2 groups of targeted fungicides effective for NCLB: FRAC Group 11 (which includes Quadris and Headline) and Group 3 (Bumper, Fitness, Proline, Propimax, AmTide Propiconazole, and Tilt). Corn is on a supplemental label for Proline. Targeted fungicides are more rain-fast than protectants and have a longer period of activity. Alternate among these groups and tank mix with a protectant fungicide to manage resistance developing in the pathogen. Starting early in disease development, when very few symptoms are present, is critical to successful control of most fungal diseases, including NCLB. The maximum number of applications that can be made to a crop is 6 for FRAC Group 11 fungicides, with no more than 2 sequential applications, and 2 to 4, depending on the product, for Group 3 fungicides. Other fungicides labeled for NCLB contain active ingredients in both FRAC fungicide groups: Headline AMP, Quilt and Stratego YLD. These are a good choice when only one or two applications are economical, as is often the case with field corn. The pre-harvest interval (PHI) is 0 days for Proline and Stratego YLD, which both contain prothioconazole. Stratego YLD also contains trifloxystrobin. PHI is 7 days for products with the other FRAC Group 11 fungicides. It is 14 days for fungicides with propiconazole as an active ingredient, which includes most of the FRAC Group 3 fungicides currently registered and the combination product Quilt. All these fungicides have a 12-hr REI.
 |
To effectively manage both NCLB and rust, growers should alternate among FRAC Group 3 and 11 fungicides, adjusting the program based on which disease is most important. Stratego is a good choice when an application is needed near harvest because the PHI is 0 days while it is 7 or 14 days for the other fungicides. Apply all targeted fungicides with a broad-spectrum fungicide for resistance management.
source : Effectively Managing Northern Corn Leaf Blight in 2013
Margaret Tuttle McGrath
Department of Plant Pathology and Plant-Microbe Biology, Cornell University
Long Island Horticultural Research and Extension Center; 3059 Sound Avenue
Riverhead, NY 11901; mtm3@cornell.edu







 Soil
insects, primarily wireworms and white grubs, can cause stand reduction
or stunted plants. These insects should be considered a serious
threat when corn will be planted in ground immediately following sod. A
preplant treatment may be considered. However, it is likely that a
planting-time treatment will provide sufficient protection.
Soil
insects, primarily wireworms and white grubs, can cause stand reduction
or stunted plants. These insects should be considered a serious
threat when corn will be planted in ground immediately following sod. A
preplant treatment may be considered. However, it is likely that a
planting-time treatment will provide sufficient protection.  Soil
insect problems generally decrease with time out of sod. Problems with
white grubs may occur in soils fertilized heavily with compost or
manure. Rootworms may cause damage in ground where corn is grown every
year. Soil insecticides will generally greatly reduce troubles with
these pests.
Soil
insect problems generally decrease with time out of sod. Problems with
white grubs may occur in soils fertilized heavily with compost or
manure. Rootworms may cause damage in ground where corn is grown every
year. Soil insecticides will generally greatly reduce troubles with
these pests.  Cutworms
Cutworms Flea
beetles are small, black, hard-bodied insects that hop or fly quickly
when disturbed. They overwinter as adults and become active early in the
spring.
Flea
beetles are small, black, hard-bodied insects that hop or fly quickly
when disturbed. They overwinter as adults and become active early in the
spring. The
real concern from flea beetles is Stewart's wilt, a bacterial disease
of corn. The pathogen is carried inside the flea beetle. Young plants
become infected as the beetles feed. Damage from Stewart's wilt is far
more severe than leaf injury caused by the beetles. Wilt resistant
sweet corn varieties should be selected to prevent losses. Chemical
control of the beetle should not be the only protection program for
Stewart's wilt.
The
real concern from flea beetles is Stewart's wilt, a bacterial disease
of corn. The pathogen is carried inside the flea beetle. Young plants
become infected as the beetles feed. Damage from Stewart's wilt is far
more severe than leaf injury caused by the beetles. Wilt resistant
sweet corn varieties should be selected to prevent losses. Chemical
control of the beetle should not be the only protection program for
Stewart's wilt.  European Corn Borer
European Corn Borer The
first generation is most vulnerable to chemical control. Treatment
should be considered if "shot-hole" damage is apparent on 25% of the
plants and live larvae are present in the whorls. One application should
be sufficient against the first generation. Your county agent for
agriculture can give you accurate information on when to expect damage.
Treatment applied after borers have entered the plant will not be
effective.
The
first generation is most vulnerable to chemical control. Treatment
should be considered if "shot-hole" damage is apparent on 25% of the
plants and live larvae are present in the whorls. One application should
be sufficient against the first generation. Your county agent for
agriculture can give you accurate information on when to expect damage.
Treatment applied after borers have entered the plant will not be
effective.  Fall Armyworm
Fall Armyworm Fall
armyworms are smooth and green to black with three thin yellow lines
down the back. A dark stripe and a wavy yellow stripe runs along each
side. Larvae have a dark head with a white inverted "Y".
Fall
armyworms are smooth and green to black with three thin yellow lines
down the back. A dark stripe and a wavy yellow stripe runs along each
side. Larvae have a dark head with a white inverted "Y". The
corn earworm is the most serious sweet corn pest because it feeds
directly on the market product. Once worms have become established
within the ear, control is impossible.
The
corn earworm is the most serious sweet corn pest because it feeds
directly on the market product. Once worms have become established
within the ear, control is impossible. Sap Beetles
Sap Beetles Silk Clipping Insects
Silk Clipping Insects

 Umumnya
gejala berupa luka pada daun, goresan hijau sampai kuning dengan
pinggiran yang tak beraturan dan bergelombang di sepanjang tulang daun
dan juga diseluruh permukaan daun. Pada beberapa kasus, permukaan daun
akan kering dan mati dengan gejala seperti kekurangan nutrisi. Pada fase
kedua ini tidak terjadi layu seperti pada fase pertama (Shurtleff 1980;
Yang 2000; Thomas 2002 dalam Harahap. 2012)
Umumnya
gejala berupa luka pada daun, goresan hijau sampai kuning dengan
pinggiran yang tak beraturan dan bergelombang di sepanjang tulang daun
dan juga diseluruh permukaan daun. Pada beberapa kasus, permukaan daun
akan kering dan mati dengan gejala seperti kekurangan nutrisi. Pada fase
kedua ini tidak terjadi layu seperti pada fase pertama (Shurtleff 1980;
Yang 2000; Thomas 2002 dalam Harahap. 2012) Pantoea stewartii.
ukurannya kecil, tubuhnya mengkilap dan berwarna hitam, ukuran panjang
kira-kira 1/16 inch. Mampu meloncat jauh.Larvanya berukuran kecil,
berwarna putih dan tidak terlalu aktif. Ukuran maksimal larva adalah
sekitar 1/6 inch dan pada umumnya segmentasi tubuhnya tidak berpigmen.
Hanya pada bagian protorax dan abdomen terakhir yang berwarna gelap
(Cook dan Weinzierl, 2004). Bakteri tumbuh subur di dalam tubuh vektor
selama hibernasi dan kemudian menginfeksi tanaman jagung dari pohon ke
pohon selama musim tanam merupakan cara penyebaran utama di lapang
(Stack et al, 2002)
Pantoea stewartii.
ukurannya kecil, tubuhnya mengkilap dan berwarna hitam, ukuran panjang
kira-kira 1/16 inch. Mampu meloncat jauh.Larvanya berukuran kecil,
berwarna putih dan tidak terlalu aktif. Ukuran maksimal larva adalah
sekitar 1/6 inch dan pada umumnya segmentasi tubuhnya tidak berpigmen.
Hanya pada bagian protorax dan abdomen terakhir yang berwarna gelap
(Cook dan Weinzierl, 2004). Bakteri tumbuh subur di dalam tubuh vektor
selama hibernasi dan kemudian menginfeksi tanaman jagung dari pohon ke
pohon selama musim tanam merupakan cara penyebaran utama di lapang
(Stack et al, 2002)
